
Transparent, Audit-Ready AI Insights in Pharma Using SAP S/4HANA

Ensuring Transparent and Compliant AI in Pharma with SAP S/4HANA
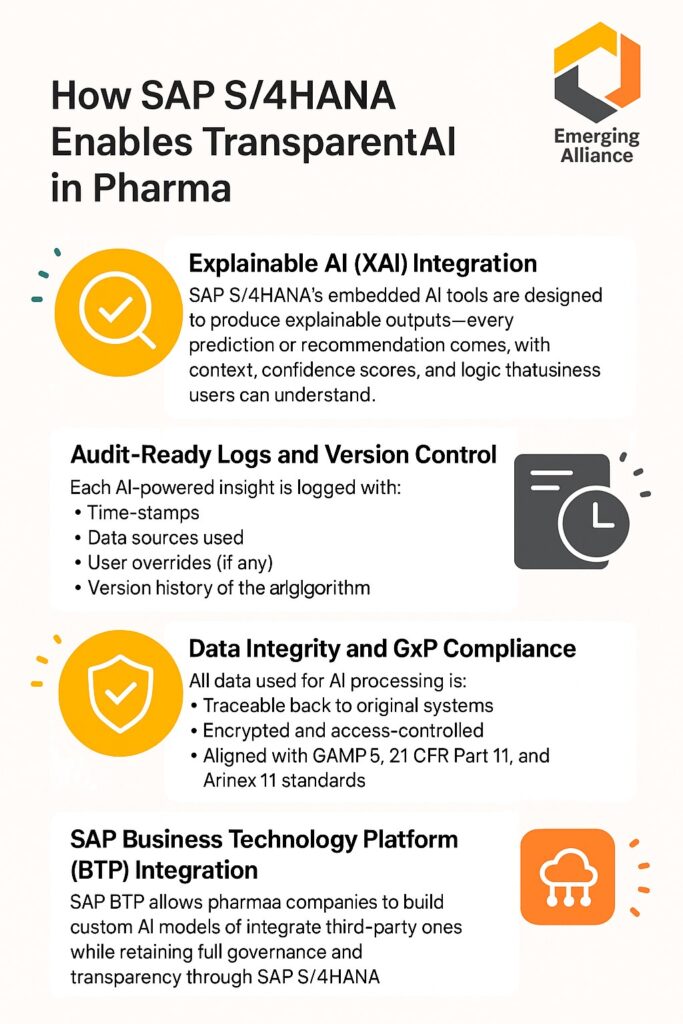
As the pharmaceutical industry accelerates toward AI-first operations, the need for transparency, explainability, and regulatory compliance has never been greater. With strict oversight from authorities like the FDA, EMA, and CDSCO, pharma companies can’t afford black-box AI models that don’t provide clarity or traceability. That’s where SAP S/4HANA steps in—delivering audit-ready AI insights with enterprise-grade transparency and data governance built in.
Why Transparency Matters in AI for Pharma
AI models are increasingly being used for:
- Predictive demand planning
- Drug formulation simulations
- Batch deviation alerts
- Clinical trial analytics
But without explainable outputs and audit-ready logs, these AI models raise red flags during inspections. Regulators demand clarity on:
- How AI made its decisions
- What data was used
- Whether human review was possible
- If the system complies with GxP and Part 11
- How AI made its decisions
- What data was used
- Whether human review was possible
- If the system complies with GxP and Part 11
Key Benefits of Audit-Ready AI in Pharma with SAP S/4HANA
| Feature | Benefit |
|---|---|
| Explainable AI | Increases trust among users and regulators |
| Centralized Audit Logs | Speeds up regulatory inspections |
| Role-Based Access Control | Prevents unauthorized model use or data exposure |
| Integrated Compliance Tools | Aligns with FDA, EMA, CDSCO, and WHO standards |
| Embedded Dashboards | Real-time visibility into AI decisions and business KPIs |
Real-World Use Case: Pharma Manufacturing Compliance
Challenge:
A pharma manufacturer using AI for batch deviation prediction struggled to justify the model’s alerts during audits.
Solution:
With SAP HANA and SAP BTP, they:
- Improved auditor trust and reduced inspection time by 35%
- Shifted to transparent AI algorithms with explainable outputs
- Created automatic audit logs with decision history
Final Thoughts
Pharma’s future is powered by AI—but not at the cost of trust, transparency, or compliance.
SAP HANA bridges that gap—offering the audit-ready infrastructure your pharma company needs to confidently adopt AI in manufacturing, R&D, clinical operations, and more.
Don’t let your AI be a black box—make it your biggest asset in regulatory compliance.
Also read, similar articles that interests you on SAP and various other ERP systems, for the Pharma industry.
FAQs
1. What is explainable AI in the context of pharma?
Explainable AI (XAI) makes AI decisions transparent, allowing users and auditors to understand how and why decisions were made.
2. Why is audit-readiness important in pharma AI tools?
Regulatory bodies require traceability, documentation, and justification for AI-driven decisions, especially in clinical and manufacturing settings.
3. How does SAP S/4HANA ensure AI transparency?
It provides explainable AI features, logs decisions, and integrates compliance tools to create transparent and audit-ready insights.
4. Is SAP S/4HANA compliant with GxP and 21 CFR Part 11?
Yes, SAP HANA follows stringent pharma compliance standards, including electronic records, audit trails, and access control.
5. Can we track which data was used in AI predictions?
Yes, SAP S/4HANA logs all data sources and parameters used in AI models, ensuring full traceability.
6. What if AI makes a wrong prediction—can we override it?
Yes, SAP allows human-in-the-loop overrides, and logs these actions for compliance and learning purposes.
7. Can I integrate third-party AI tools with SAP?
Yes, through SAP BTP, you can safely connect third-party AI while keeping audit logs and security intact.
8. Does SAP offer dashboards for AI insights?
Absolutely. SAP Fiori and SAP Analytics Cloud offer visual dashboards for real-time monitoring of AI-driven decisions.
9. Is SAP S/4HANA suitable for small to mid-sized pharma companies?
Yes, it’s scalable and available in both private and public cloud models for companies of all sizes.
10. How do I start implementing audit-ready AI in my pharma company?
Partner with a certified SAP provider like Emerging Alliance to assess, implement, and scale AI with compliance in mind.
🌐 Ready to map the future of your Pharmacovigilance operations?
Let Emerging Alliance help you integrate AI + SAP for a smarter, safer future. 📩 Schedule a Free Demo







